Canine mast cell tumours: the great pretender
Mast cell tumours (MCT) are the most common skin tumour, comprising 16-21% of cutaneous tumours in the dog. They may affect any breed, but Boxers, Boston Terriers, and Labrador Retrievers are reportedly predisposed, and older dogs (average 9 years) are most often affected. The etiology is unknown but chronic skin inflammation may be a predisposing factor. Although the genetic alterations that predispose some dogs to MCTs are not completely understood, research has identified expression of c-kit, a tyrosine kinase receptor. Several studies have demonstrated the presence of mutations in c-kit and based on this new information, novel therapies are now available to treat MCTs.
Clinical presentation
• Great imitators – they can look like anything!
• Variable: dependent upon form of MCT
• Typically just dermal or subcutaneous mass noted - often an incidental finding
• GI signs may occur secondary to degranulation (histamine release)
• Swelling and/or bruising secondary to degranulation (histamine and heparin release)
Lesions are most often single, firm nodules, but multiple lesions are seen in 5-15% of patients
• Well to moderately differentiated tumours tend to be solitary, slow growing, and firm to fluctuant in texture
• Poorly differentiated tumours may have more aggressive histories, with rapid growth, potentially with intermittent tumour swelling and pain
Diagnostic evaluation and clinical staging
A practical approach based on clinical judgment, together with cytological evaluation, is recommended regarding whether to stage prior to or after tumour removal. The minimum staging tests that should be done prior to tumour removal include standard pre-anesthetic bloodwork, fine needle aspirate (FNA) of the regional lymph node, thoracic radiographs, and perhaps abdominal ultrasound. Although MCTs rarely metastasize to the lungs, a thoracic radiograph will help rule out the presence of an unrelated disease process (or even a second tumour). Bone marrow aspirate and buffy coats may be reserved for later.
Fine needle aspirate with cytology
• Always the first step in diagnosis and surgical planning – these tumours exfoliate well!
• FNA mass plus regional lymph node (even if LN is normal size and texture)
• Bleeding/swelling post aspirate are common
• Cytology reveals (Figure 1):
• Uniform population of medium sized round cells with metachromatic granules
• Granules are acidic, so stain with basic dyes (e.g. giemsa, toludine blue, methylene blue). May not stain with DiffQuick
• Eosinophils may be seen
• Mast cells may be found in normal lymph nodes – look for clusters and/or “anaplastic” mast cells to increase suspicion of lymph node metastasis
Figure 1: Cytology of a fine needle aspirate of a well differentiated MCT
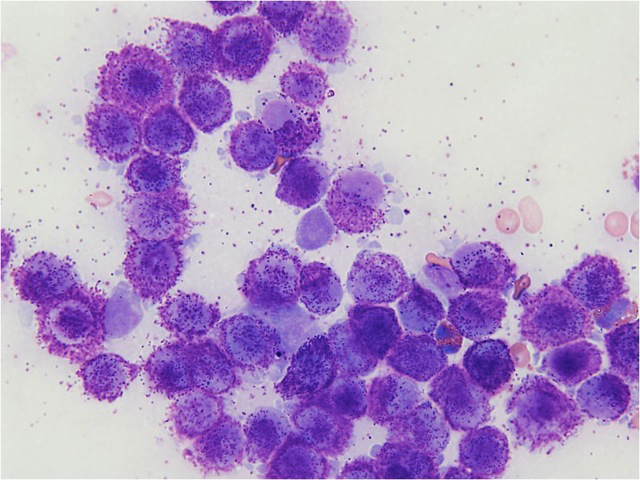
Photo credit: Dr. Ryan Dickinson, DVM, DACVP
Bloodwork (CBC/Chemistry) may often be normal, though anemia may be present if there’s severe GI ulceration/bleeding. Peripheral eosinophilia or basophilia on CBC, and circulating mastocytosis is rare and is usually associated with visceral MCTs.
Abdominal imaging may reveal infiltrative disease. Aspiration of normal liver/spleen is usually unrewarding and actually may complicate the case as the presence of nonmalignant mast cells in these organs is a normal finding.
Bone marrow aspirate will reveal a low incidence of MCT in the marrow (~4%).
Thoracic radiographs can be useful to assess sternal lymph node (drains abdomen) and to evaluate for other intrathoracic disease prior to therapy.
Buffy Coat Smear is non-specific and insensitive, and dogs with inflammatory or allergic diseases will have highest numbers of mast cells in the buffy coat.
Biopsy
• Diagnostic and therapeutic
• Excisional preferred unless diagnosis is unclear
• Assess grade of tumour
• Assess margins
• Patnaik grading system (used in North America)
• Grade 1 = well differentiated
• Grade 2 = moderately differentiated
• Grade 3 = poorly differentiated
•Proposed new 2-tier histologic grading system (high versus low)
Mast cell tumour panel
The mast cell panel consists of cell proliferation analysis (Ki-67, PCNA and AGNOR), c-Kit PCR and KIT immunohistochemistry. Results of this panel are all linked to MCT-associated mortality and survival times. While there is some association between each independent test, prognoses developed from interpretation of all three analyses offer clients the highest level of certainty.
Treatment options
Decisions in how to treat MCTs are dependent on the clinical stage of disease and whether or not negative prognostic indicators are present.
Surgery is the treatment of choice for all MCTs unless the tumour is impossible to remove. Previously, wide surgical margins of 3 cm were recommended but it has been found that 2 cm margins may be adequate for grade 1 and 2 tumours. Three centimeter margins are still recommended for grade 3 tumours. However, most times you will not know the grade prior to definitive surgery, so take 3 cm if possible and one fascial plane deep. Remember to ink the margins.
Clean vs. dirty excision?
• Local recurrence for completely excised grade 2 MCTs is 5-11%
• At 1 and 2 years post-op, incidence of local recurrence is increased with dirty margins
• Complete excision has been associated with prolonged survival
• Complete excision of grade 1 and 2 tumours will most likely be curative
• Exception: Metastatic disease
• Complete excision of grade 3 tumours will most likely prevent local recurrence
• BUT, chemotherapy is ALWAYS indicated due to high incidence of metastasis
Radiation therapy (RT) is another local treatment for MCTs as they are very sensitive to radiotherapy. RT is most commonly used for localized tumours that have been incompletely excised from areas where a second surgery is impossible (e.g. on a limb).
Chemotherapy is indicated for ALL grade 3 MCTs and ALL metastatic grade 1 or 2 MCTs. Chemotherapy may also be used for gross non-resectable disease or disease in an unfavourable location or it may be used in place of radiation therapy when RT is not an option. Several drugs have been used with varying response rates/durations of response.
Ancillary therapy
• H1 antagonists – diphenhydramine (Benadryl®)
• Block systemic signs of degranulation
• H2 antagonists – famotidine (Pepcid®), ranitidine (Zantac®), cimetidine (Tagamet®)
• Prevent/control GI ulceration
• 35 to >80% of dogs with MCTs have been reported to have subclinical GI ulceration
• For clinical GI ulceration (e.g. melena), may need to add sucralfate and/or omeprazole
Tyrosine kinase inhibitors
Presently there are two small molecule inhibitors available in the veterinary market for use in MCTs. Toceranib (Palladia) inhibits KIT, PDGFR, and VEGFR with strong similarities to multi-targeted tyrosine kinase inhibitors in humans. Mastinib (Kinavet) inhibits KIT and PDGFR. Because the prevalence of Kit mutations in canine MCTs is approximately 9-30% and higher grade tumours are more likely to have a mutation, it was reasoned that these tumours would likely respond to these small molecule inhibitors.
Prognostic factors
The most useful predictor of survival is the histological grade. Even with the newly proposed 2-tier grading system, high-grade MCTs were significantly associated with shorter time to metastasis or new tumour development and with shorter survival time. The median survival time was less than four months for high grade MCTs but more than two years for low grade MCTs.
C-Kit mutation status appears to be a very important predictor of response to therapy (Palladia). Several studies have shown that this mutation appears to be associated with a higher grade and/or more aggressive behaviour. Mitotic index (MI) appears to be a strong predictor for survival. In a 2007 study, the median survival time for dogs with a MI less than 5 was significantly longer (70 months) than for those with an MI greater than 5 (2 months), regardless of grade. One recent study evaluating subcutaneous tumours reported that decreased survival time was linked to mitotic index, infiltrative growth pattern, and the presence of multinucleation.